📢 New review article published in Protein Science!
🧪 Our latest review is finally online in Protein Science!
🎉 We’re excited to share that our review article, “Experimental methods for studying amyloid cross-interactions”, is now published and open access in Protein Science!
🧬 What’s inside?
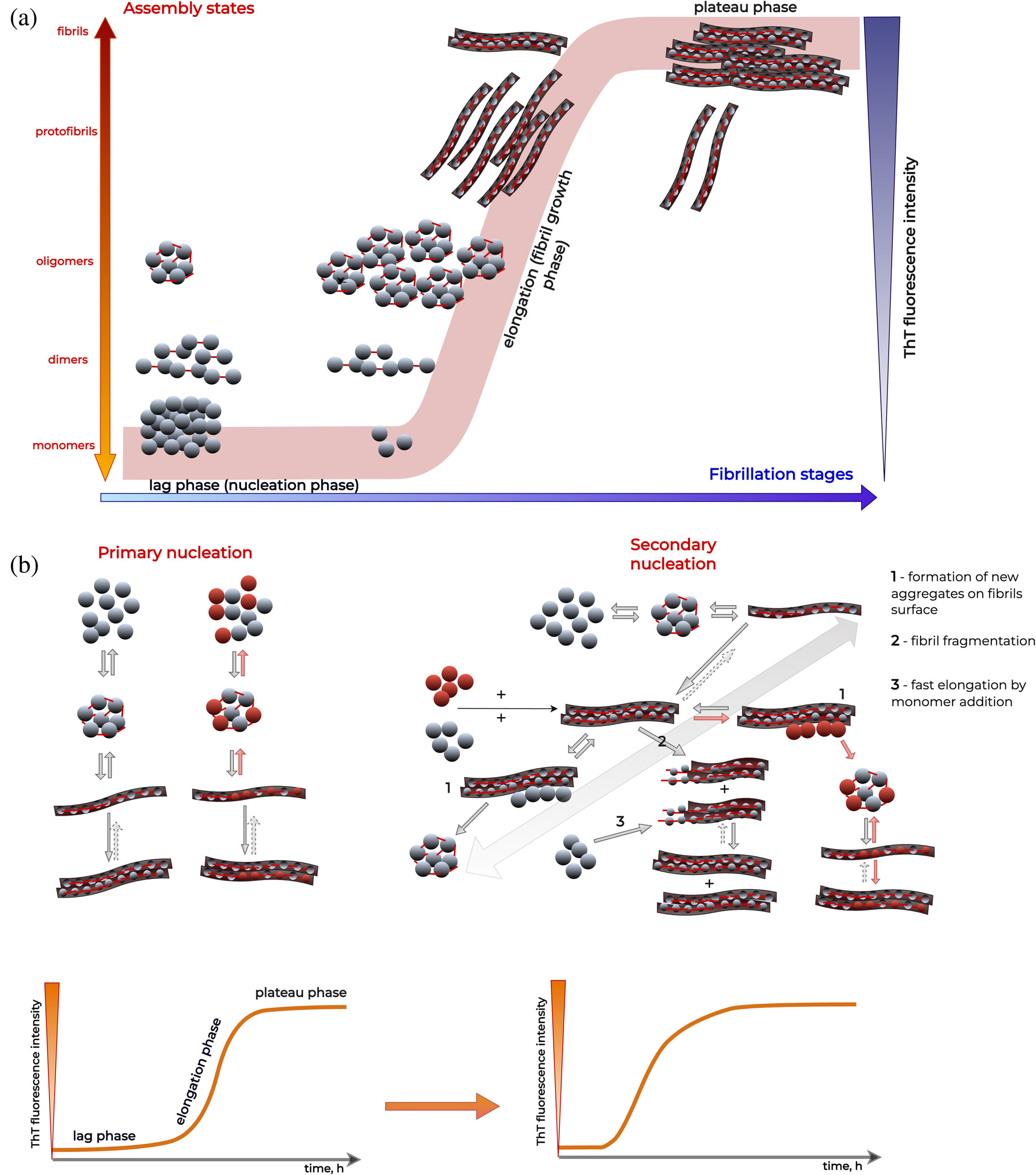
Cross-interactions between amyloid proteins are a key factor in many diseases, from Alzheimer’s to prion diseases. Yet, capturing the full picture of these interactions remains a major experimental challenge.
In this comprehensive review, we:
- 🔬 Survey the most widely used (in vitro) and in vivo methods to study amyloid cross-interactions
- 💡 Discuss how each method contributes unique (but partial) insights, from aggregation kinetics to fibril morphology and hetero-aggregate composition
- 📷 Highlight the complementarity of techniques such as:
- Thioflavin T fluorescence
- AFM, Cryo-EM, immuno-TEM
- Mass spectrometry & solid-state NMR
- Co-immunoprecipitation and super-resolution imaging
📊 And we emphasize: no single method is enough, it’s only through hybrid strategies that we can start to decode the complexity of amyloid cross-interactions.
📌 Key takeaway:
“The only recommended approach is to combine indirect and direct evidence to create a more complete outlook of this process.”
Whether you’re developing new tools, studying amyloid polymorphism or exploring proteinopathies, we hope this work helps chart the path toward better experimental designs and interpretations.
🧠💪 Congrats to the whole team and special thanks to everyone who contributed ideas, figures and hard-won insights!
